Abstract
Introduction Diffuse large B-cell lymphoma (DLBCL) is a disease with high molecular and genetic heterogeneity, giving challenges to research and therapy in relation to prognosis. Ferroptosis, a form of regulated cell death, is produced by excessive lipid peroxidation. Induction of ferroptosis may be a promising therapeutic strategy contributed to triggering cancer cell death. However, the function of ferroptosis in DLBCL and its under mechanism are still undefined, especially the interaction with the tumor immune microenvironment (TIME). Here, we aimed to systematically investigate novel prognostic biomarkers by ferroptosis-related genes (FRGs) and explore their connection with TIME in DLBCL.
Methods RNA sequencing (RNA-seq) data and corresponding clinical information of DLBCL patients (n=928) were analyzed. The differential expression of FRGs was determined and the prognostic risk signatures were analyzed. Kaplan-Meier survival analysis, univariate and multivariate Cox regression analyses and receiver operating characteristic (ROC) curve analysis were performed. The potential functions of FRGs in DLBCL were explored using GO, KEGG enrichment and single-sample gene set enrichment analysis (ssGSEA). The immunoscore was calculated utilizing the ESTIMATE algorithm.
Results To evaluate the effect of ferroptosis genes in DLBCL progression, we systematically explored the expression patterns of 60 FRGs and found that most of them were dysregulated. Subsequently, the effect of ferroptosis genes in the clinical prognosis of DLBCL patients was probed. 10 genes were identified based on the LASSO algorithm to calculate the risk score. The low-risk group showed significantly prolonged OS in DLBCL patients. Furthermore, to evaluate the prognostic accuracy of the risk signatures, we performed 1-, 2-, and 3-year ROC curve analyses and the respective AUC values displayed the reliability of the prediction.
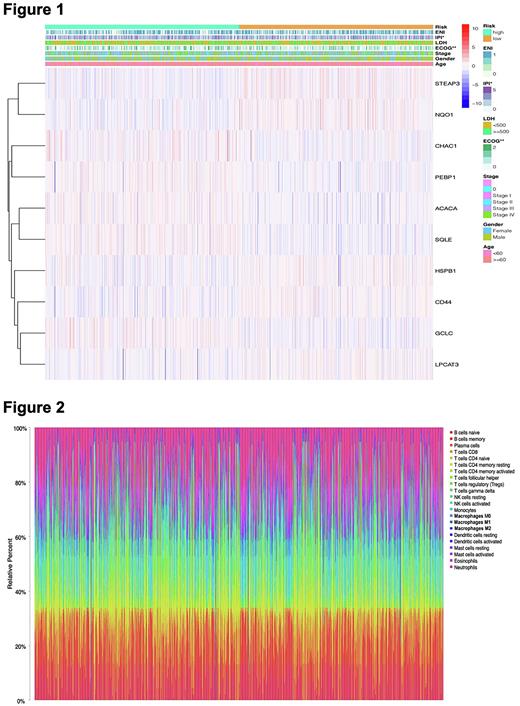
The clinicopathological features between high-risk group and low-risk group were next evaluated, risk score was shown a significant relevance to IPI and ECOG (p<0.05; Figure 1). Univariate and multivariate Cox regression analyses indicated that risk signature was implicated in OS and can be identified as an independent indicator in DLBCL patients.
GO and KEGG enrichment analysis were used to probe the biological function of ferroptosis genes and the results revealed the remarkably enriched in inflammatory cell activation, immune response, and PD-L1 checkpoint pathway. Therefore, we speculated that ferroptosis can modulate the composition of the TIME, and then assessed the enrichment degrees of immune cells and the activity of immune-related pathways between high- and low-risk groups through the ssGSEA. Subsequently, CIBERSORTx was utilized to calculate the abundance ratios of 22 types of immune cells for exploring the microenvironment components of DLBCL patients (Figure 2). The immune cell interaction in TIME was observed by the correlation coefficient heatmap. We finally found that the immunoscore of the low-risk group and the expression levels of PD-L1, a vital immune checkpoint, were higher than those of the high-risk group.
Conclusions We identified that ferroptosis genes were dysregulated and ferroptosis genes-based risk score was an independent prognostic indicator of DLBCL patients. More importantly, our study indicated that ferroptosis genes-based risk signatures were remarkably related to the immune cell infiltration levels, and ferroptosis might participate in the regulation of DLBCL immune microenvironment. Further assessment of the ferroptosis patterns in DLBCL may improve the comprehension of immune infiltrations, contributing to achieving individualized immunotherapeutic strategies for this patient population.
Keywords: ferroptosis, prognosis, tumor immune microenvironment, immunotherapy, DLBCL
Disclosure No conflicts of interest to declare.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.